Driving growth and performance in Bio-Pharma businesses
UK & EU Market Entry for US FDA Approved Sterile Injectables
Turnkey UK & EU Market Entry for Sterile Injectables
If you hold an approved US FDA ANDA for a small-molecule sterile injectable, Oakley Business Development provides a structured, low-risk route to UK or EU market entry.
Our turnkey program begins with mandatory regulatory and technical due diligence, then guides your product through strategy selection, technology transfer, validation, and commercialization — fully aligned with MHRA and EMA requirements, including EU FMD serialization.
You retain ownership and control throughout, while Oakley acts as your single, accountable partner from review to launch.
Every Successful UK or EMA launch starts with an evidence-based go / no-go decision.
Unlock New Revenues from Your FDA ANDA in the EU/UK
Turn existing IP into commercial success.
We guide the entire pathway, end-to-end
A Proven Pathway to Market Entry
At Oakley Business Development, we’ve perfected a stepwise pathway that turns a US-approved sterile injectableinto a launch-ready product for the UK and EU. Every stage is built to reduce risk, speed approvals, and unlock the full value of your portfolio.
The Oakley Process
Assess. We conduct deep regulatory and technical due diligence on your ANDA CTD — exposing gaps, regional nuances, and the best MHRA or EMA route.
Decide. Your Oakley Due Diligence Report delivers clear timelines, costs, and opportunities so you can move forward with confidence.
Deliver. Select from three fully costed Market Entry Tracks tailored to your strategy — with optional services for a true turnkey program.
Launch. Your product exits the process ready for submission, validated manufacture, or full market launch — unlocking new revenue across the UK and EU.
Every Successful UK or EMA launch starts with an evidence-based go / no-go decision.
Unlock New Revenues from Your FDA ANDA in the EU/UK
Turn existing IP into commercial success.
We guide the entire pathway, end-to-end
From Review to Launch – Step by Step
Evaluation: The Foundation of Success
Every project starts with Oakley’s Due Diligence Review — a deep dive into your existing ANDA CTD.
We pinpoint dossier gaps, regional differences, and market potential, then map the optimal route — UK National Assessment, EU National/Decentralized, or Centralized procedure.
This independent, mandatory first step forms the foundation of the Oakley Process.
Your Due Diligence Report delivers:
A commercial assessment of your product in the UK and EU hospital markets
Reference drug confirmation
A precise gap analysis of your ANDA
The outcome: a clear, evidence-based recommendation that drives a confident go/no-go decision and selection of your development Track.
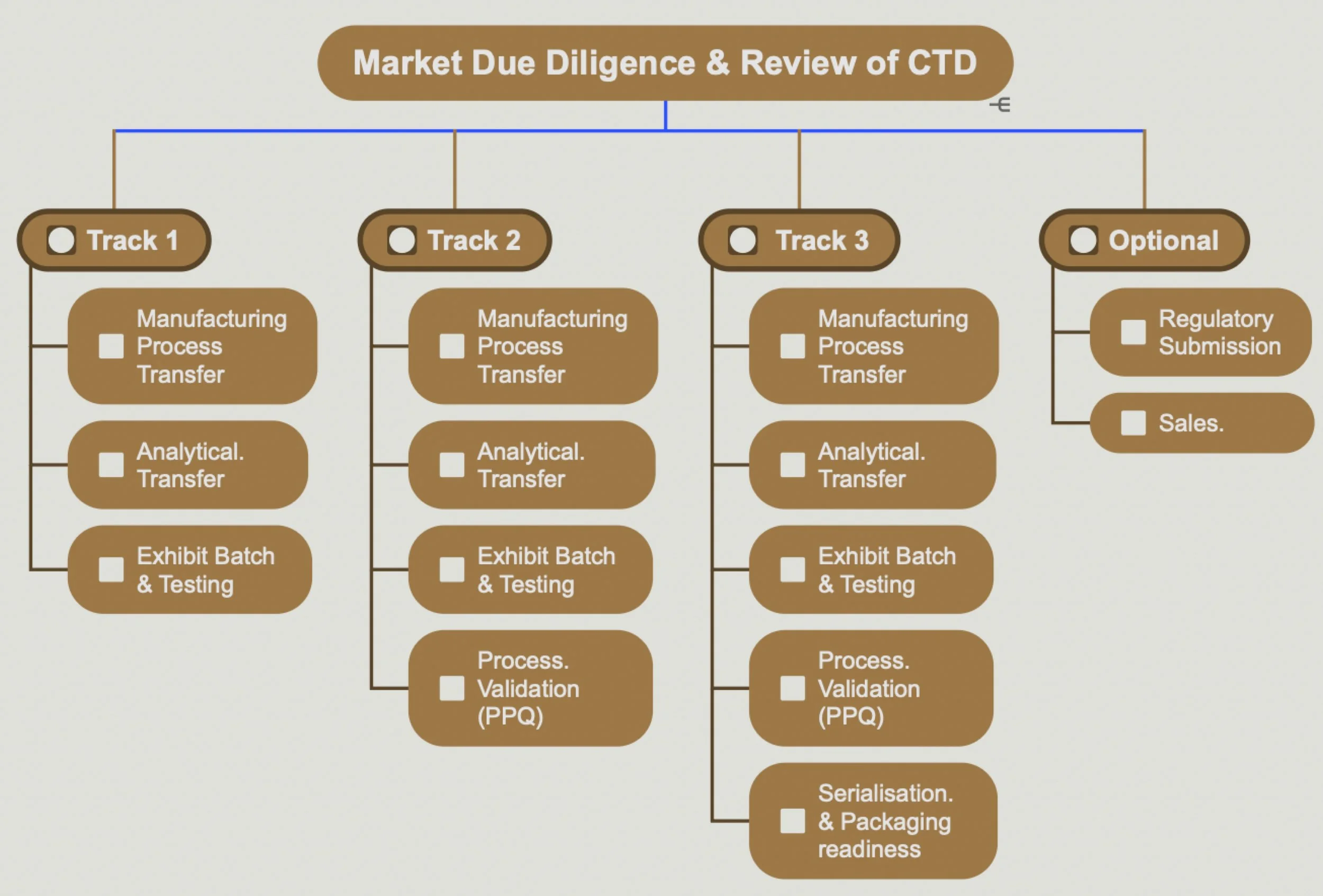
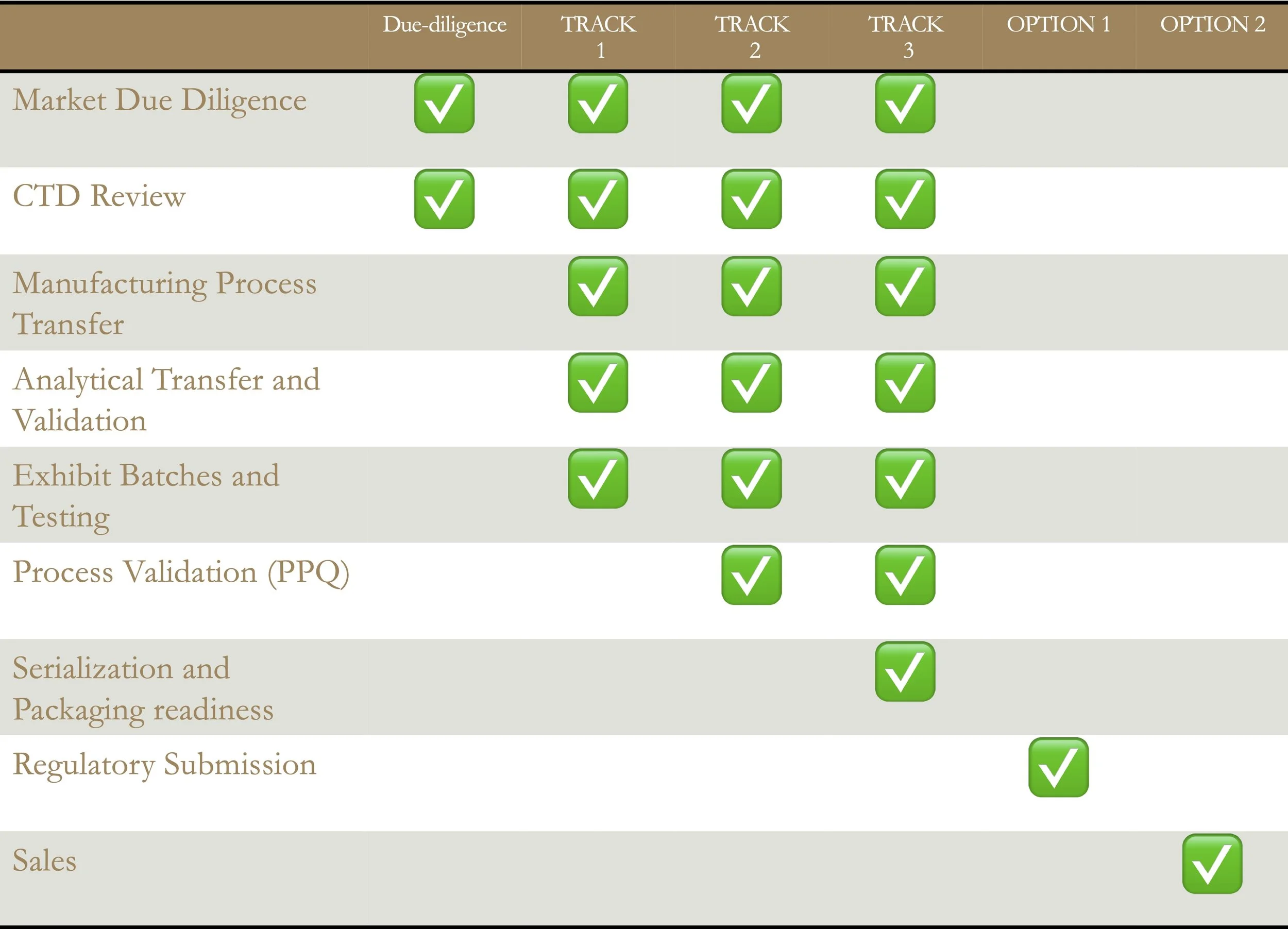
Track 1 - Transfer & Regulatory Readiness
We transfer your manufacturing process and validated analytical methods to an EU/UK facility — aligning with MHRA and EMA standards.
Track 1 concludes with Exhibit Batch manufacture and testing, delivering a complete data package ready for regulatory submission.
Track 2 – Validation & Commercial Preparedness
Track 2 builds on Track 1 with Process Validation Batch Manufacture to prove reproducibility and compliance.
Where permitted, validation batches are configured for sale, maximizing efficiency and return on investment.
Track 3 – Serialization & Launch Readiness
Track 3 includes all prior steps and adds Serialization and Packaging Readiness to ensure full EU FMD compliance.
Activities are tailored to your existing serialization and packaging partnerships — delivering a launch-ready product.
Optional Services Enhance your program with Oakley’s value-add support, including:
Regulatory submission preparation (MHRA/EMA)
Sales and market entry support in selected territories
Flexible, on-demand services aligned with your business goals — providing a truly turnkey route to market success.
Every Successful UK or EMA launch starts with an evidence-based go / no-go decision.
Unlock New Revenues from Your FDA ANDA in the EU/UK
Turn existing IP into commercial success.
We guide the entire pathway, end-to-end
Tech-Transfer, Validation and Commercialization
Oakley BD manages every step of your technology transfer and validation process, ensuring a smooth transition from US manufacturing to compliant, in-market EU or UK production.
Our team coordinates process transfer, analytical validation, and PPQ execution to meet MHRA and EMA expectations — minimizing disruption and accelerating readiness for approval.
From there, we guide your commercial manufacturing strategy, aligning supply chain, packaging, and market launch activities so your sterile injectable product moves seamlessly from approval to availability.
Optional Services
Enhance your program with Oakley’s value-add support
Oakley streamlines your regulatory submission — preparing complete MHRA and EMA dossiers using data from your development track. Our team ensures your submission is fully compliant, expertly formatted, and ready for seamless electronic delivery.
Oakley supports your hospital sales strategy with a tailored, market-specific solution designed to maximize the commercial potential of your product portfolio in your chosen territory.
Why Choose Oakley?
Over 30 years of global pharma and CMC expertise deploying complex product transfers across the US, Europe, and Middle East
Deep understanding of MHRA, EMA, and EU FMD requirements — we design strategies that regulators respect
Integrated dossier-to-launch service: from due diligence to commercialization without handoffs or gaps
Fully costed Track options tailored to your timeline, risk tolerance, and budget
Proven tech transfer and validation capability — bridging US systems to compliant European operations
Commercial readiness built in: launch packaging, manufacturing scale-up, and market strategy
Flexible value-add services (sales, regulatory support) to boost market success
Single accountable partner across technical, regulatory, and commercial domains
Every Successful UK or EMA launch starts with an evidence-based go / no-go decision.
Unlock New Revenues from Your FDA ANDA in the EU/UK
Turn existing IP into commercial success.
We guide the entire pathway, end-to-end
Frequently Asked Questions
Every Successful UK or EMA launch starts with an evidence-based go / no-go decision.
-
A single, streamlined pathway that takes an FDA‑approved sterile injectable from US approval to UK/EU market readiness. It covers due diligence, technology transfer, analytical validation, regulatory submission, serialization, and commercialization support.
-
Assessment of your existing ANDA CTD for completeness and regional differences, confirmation of reference product, commercial opportunity review, and a gap analysis that maps the optimal MHRA (UK) and/or EMA (EU) route.
-
Yes. It is an independent, fixed‑fee foundation step. The report is yours to keep and does not obligate either party to proceed; it informs a clear go/no‑go and the choice of development Track.
-
Timelines vary by dossier readiness, product complexity, and pathway. As a guide, many programs complete in ~12–18 months from due diligence to submission/launch, with some extending to ~24 months.tem description
-
This turnkey program is focused on small‑molecule sterile injectables for hospitals. Oakley has experience across other dosage forms and pathways, which can be supported on a bespoke basis outside this program.
-
No. This is a fee‑for‑service program. You remain the MA applicant/holder; Oakley does not seek license rights, profit shares, or ownership of the MA. Under Tracks 2/3, commercial supply is typically on a price‑per‑unit basis.
-
Tracks are delivered in discrete segments. You may pause at segment boundaries, truncate a Track after a completed segment, or escalate (e.g., from Track 1 to Track 2) via change order.
-
Additional products can be added under a Master Agreement via change order, or set up under separate agreements if preferred.
-
Yes. Data generated is compatible with MHRA and/or EMA submissions. UK approval covers the UK. EMA approval enables EU/EEA marketing (subject to local pricing/market access). Your territory plan can be confirmed after Track 1 without disrupting the program.
-
At qualified EU/UK facilities with sterile injectable expertise. Tech transfer, method transfer, PPQ, and related validation work are completed under cGMP and aligned with MHRA/EMA expectations.
-
Yes. Oakley prepares compliant dossiers using Track data, manages eCTD publishing and electronic submission, and supports authority interactions as required.
-
Yes. Track 3 includes serialization and packaging readiness designed to meet EU Falsified Medicines Directive (FMD) and local requirements. We can coordinate with your providers or recommend qualified partners.
-
A local legal presence/agent is required by authorities. This is outside the core program scope, but Oakley can introduce potential agents if needed.
-
Potentially, if the site meets applicable EU/UK GMP and Qualified Person (QP) release expectations. If not, in‑market transfer may be required. Oakley will advise the optimal approach during due diligence.
-
No. An ANDA can support EU/UK filings as a data reference, but approvals are separate and not automatically recognized by each authority.
-
Yes. MA transfer to another legal entity is possible via authority procedures. Oakley can advise on process and sequencing alongside your legal team.
-
No vendor can guarantee approvals. Oakley’s role is to identify gaps, generate compliant data, and prepare high‑quality submissions; outcomes depend on independent authority review.